University of Thi-Qar … College Of Science ……….. Department of Chemistry Organic Chemistry Second Stage Lecture 1 ( B

SOLVED: In valence bond theory, which orbital hybridization combination is found in O=C=O? A. 2s in carbon with a 2s in oxygen B. 2p in oxygen with a 2p in carbon C.

For each atom listed, identify the geometry as one of the following: tetrahedral, square planar, trigonal planar, trigonal pyramidal, or linear. | Homework.Study.com
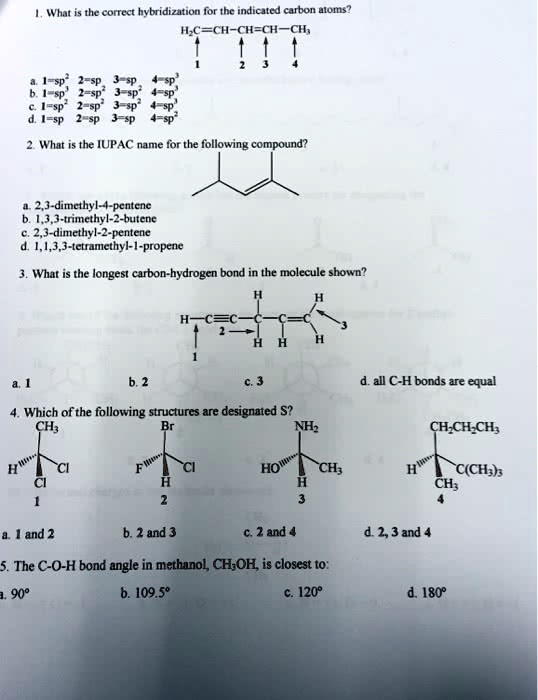
SOLVED: 1. What is the correct hybridization for the indicated carbon atoms? H2C=CH-CH=CH-CH3 a. -sp2, 3-sp3, 4-sp2. 2. What is the IUPAC name for the following compound? a. 2,3-dimethyl-4-pentene b. 1,3,3-trimethyl-2-butene c.
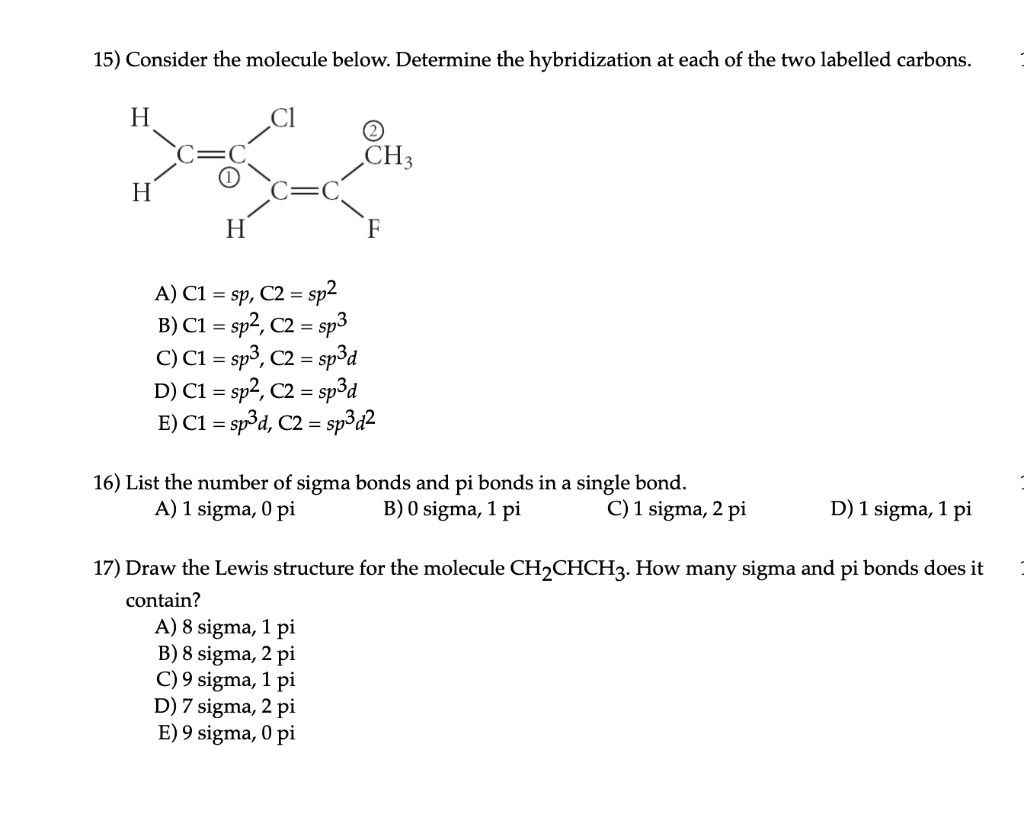
SOLVED: 15) Consider the molecule below. Determine the hybridization at each of the two labeled carbons. H 2 H H A) C1 = sp, C2 = sp2 B) C1 = sp2, C2 =

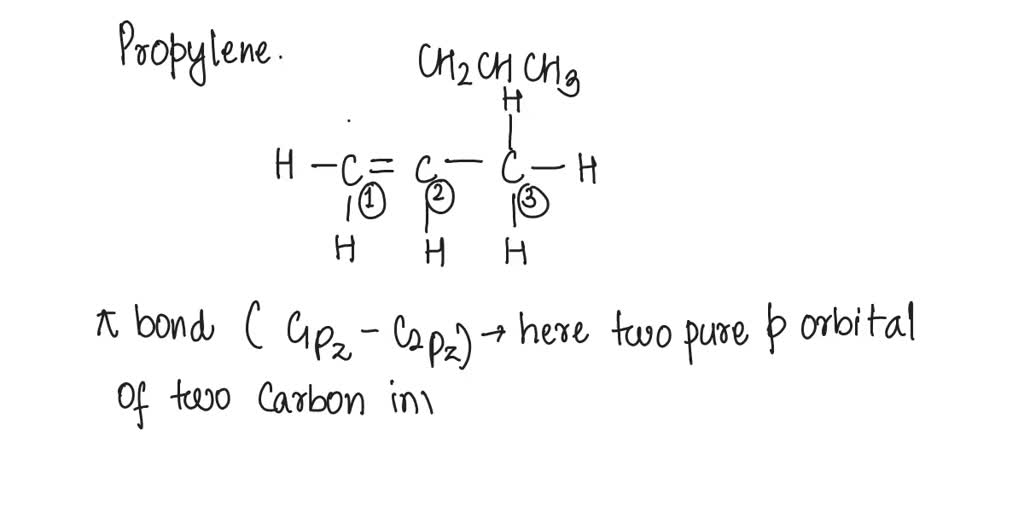
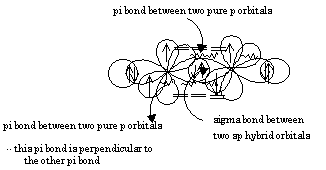
Draw the Lewis structure for the molecule CH2CHCH3. How many sigma and pi bonds does it contain? | Homework.Study.com
Write the type of hybridisation of each of the carbon atom in the following structures: (i) ch2=c=ch2 (ii) ch3 ch=ch ch3

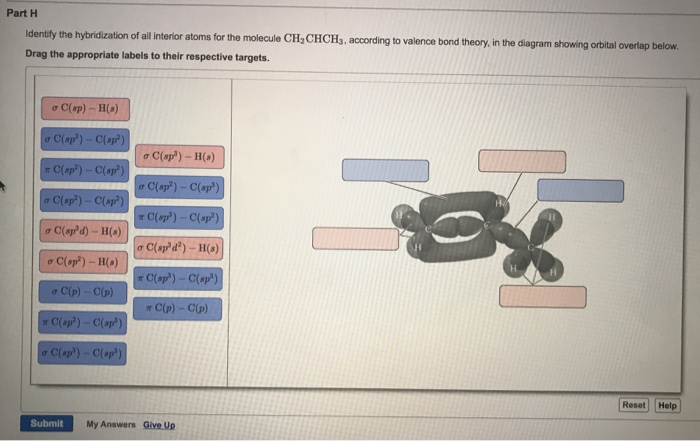
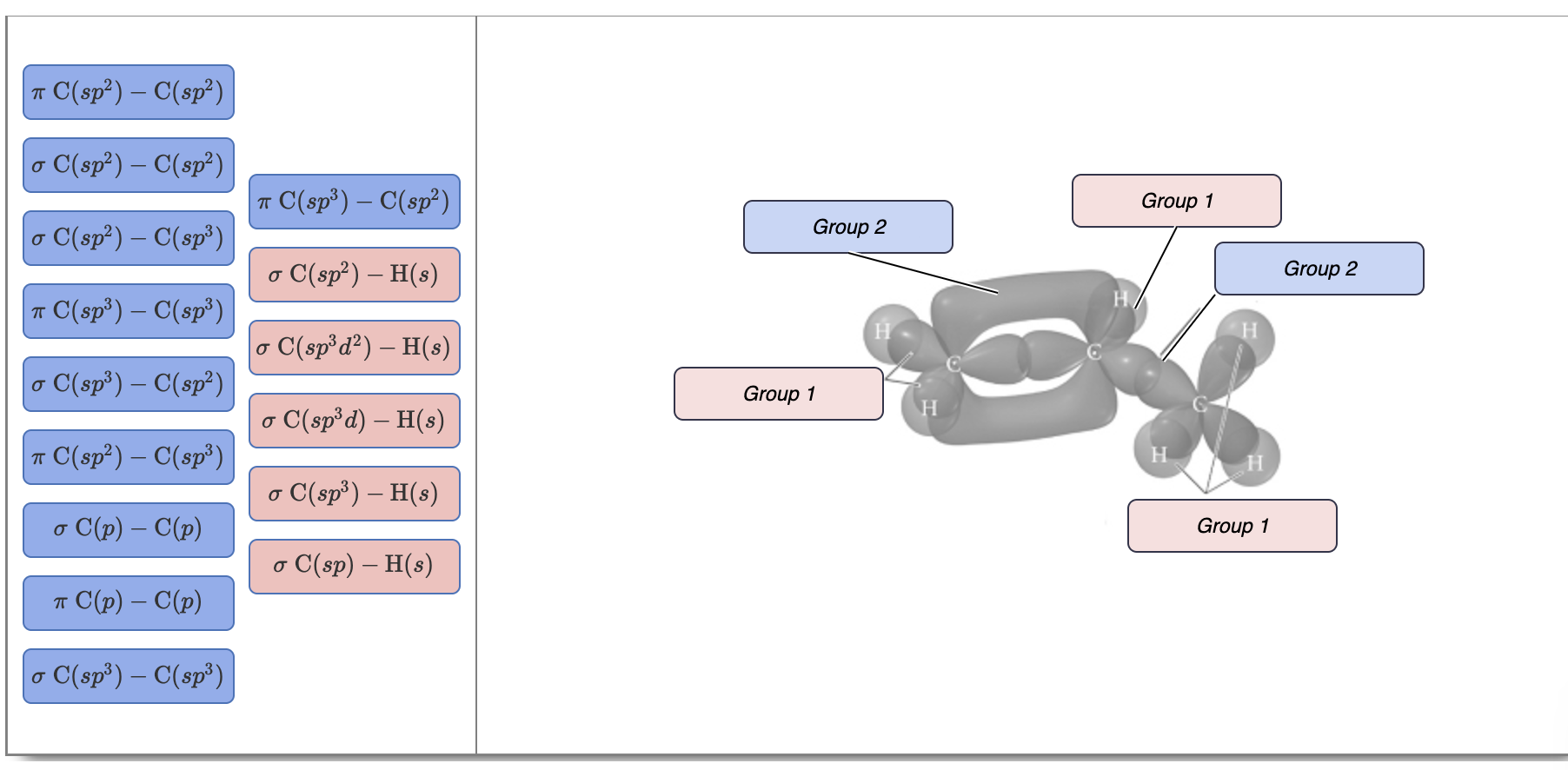
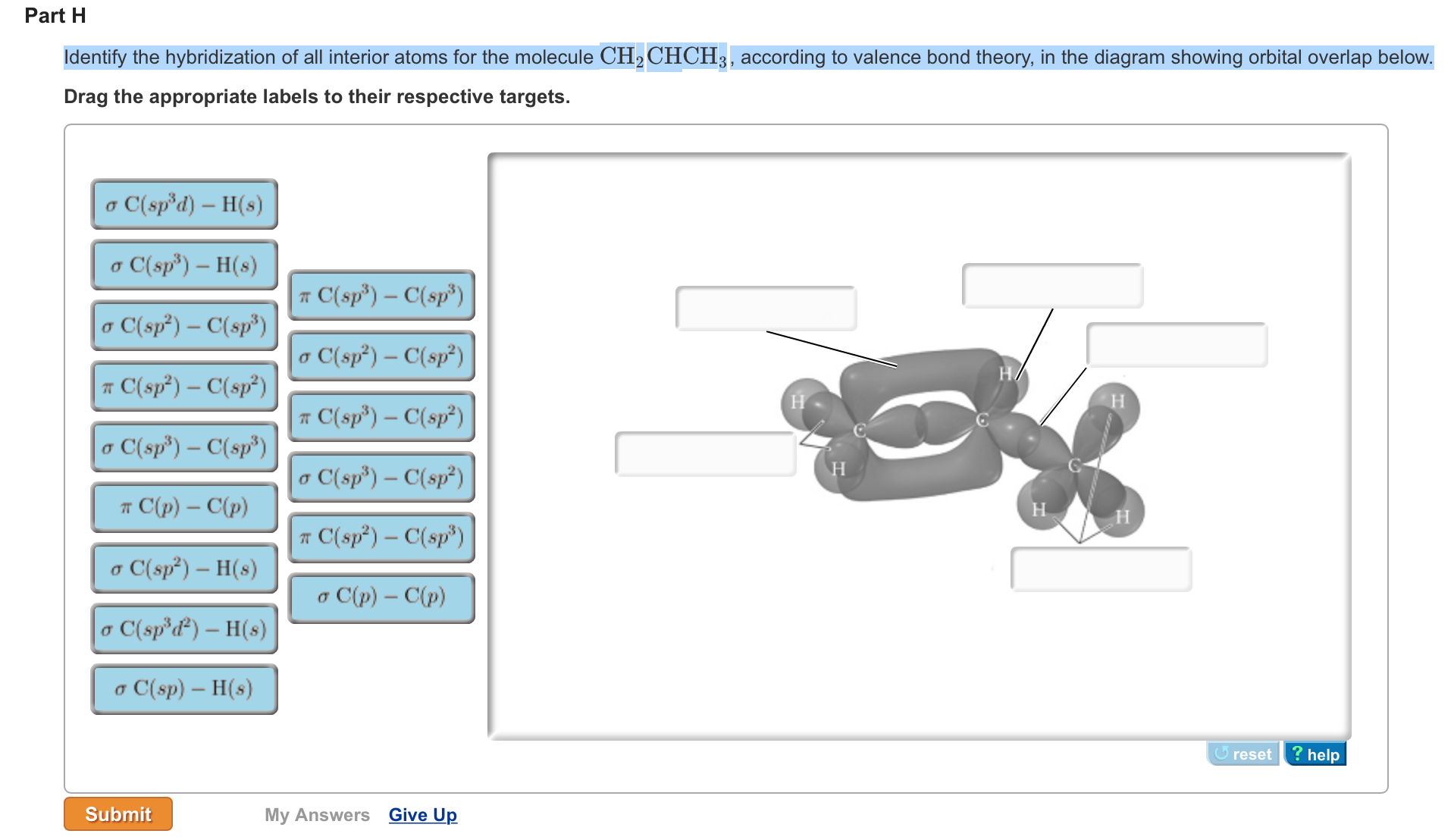
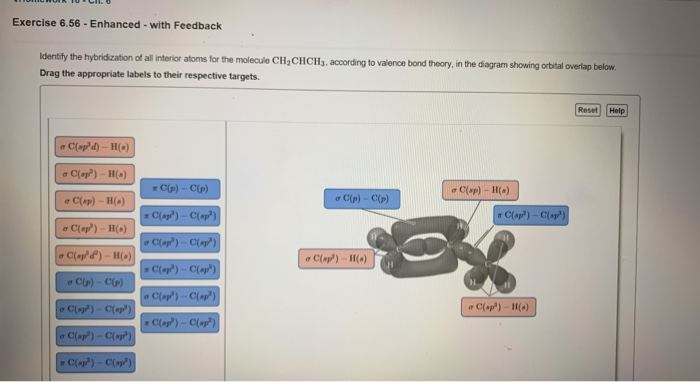
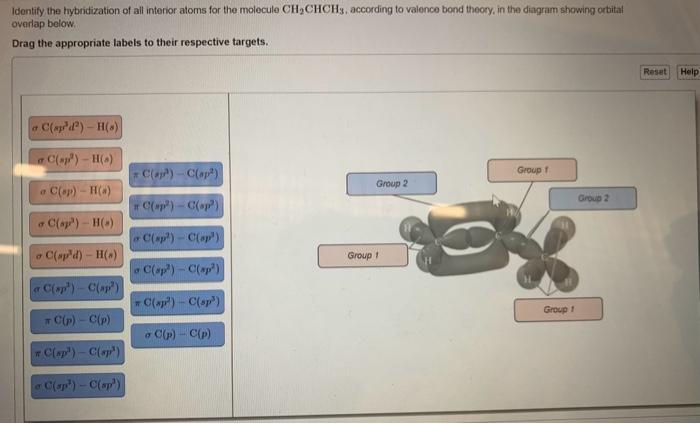
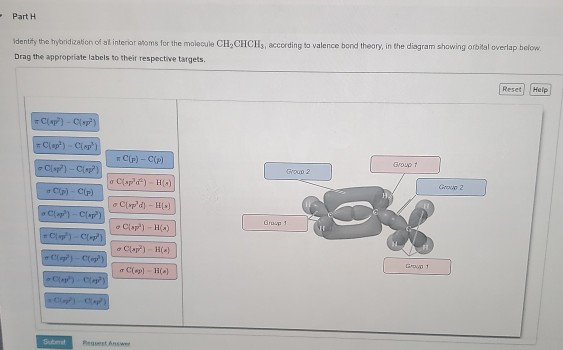
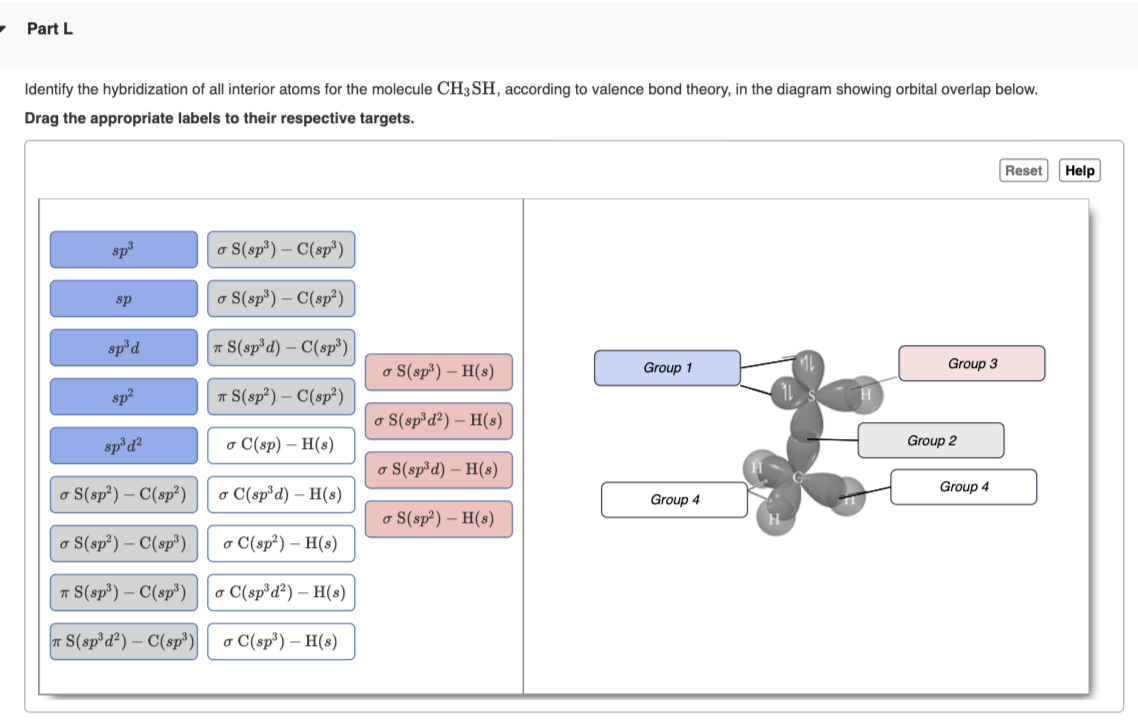
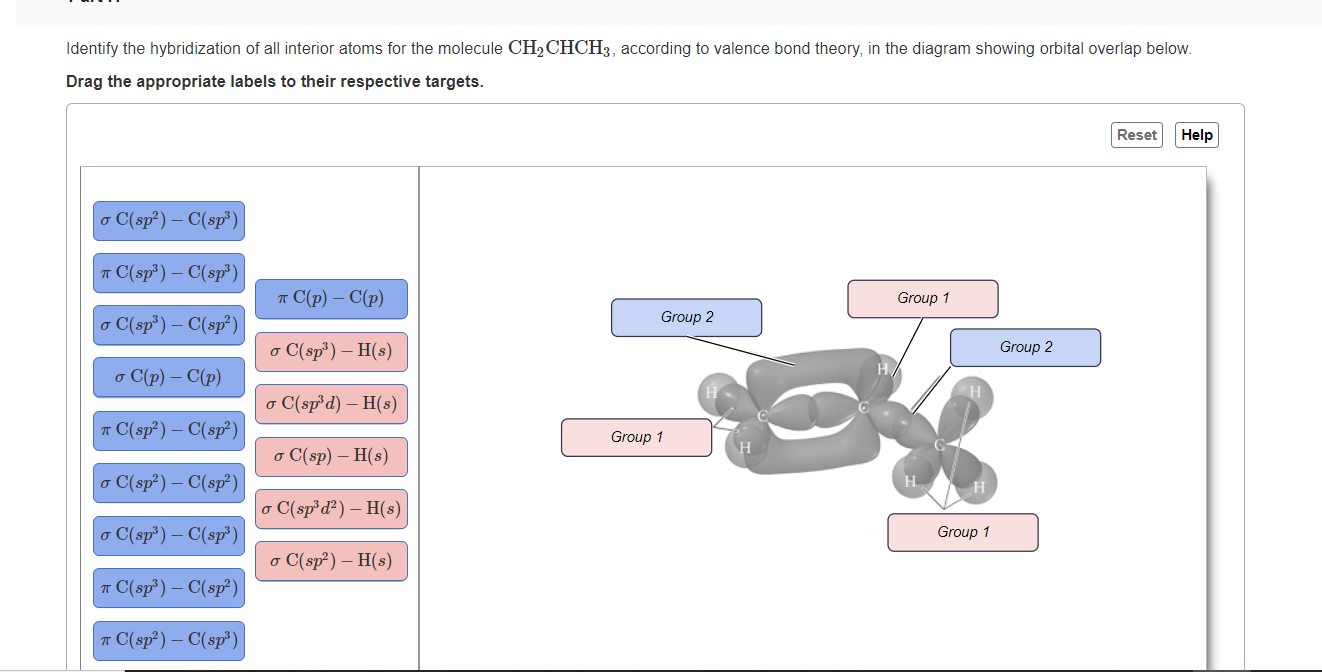
OneClass: Part H Identify the hybridization of all interior atoms for the molecule CH2 CHCHs, accordi...

Explain what is meant by the term "hybridization" in molecular orbital theory and show how the concept can be used to explain the structure and bonding in ethane C2H6, ethene C2H4, and

Draw the lewis structure for the molecule ch2chch3. how many sigma and pi bonds does it 30) contain - brainly.com

Draw the lewis structure for the molecule ch2chch3. how many sigma and pi bonds does it 30) contain - brainly.com