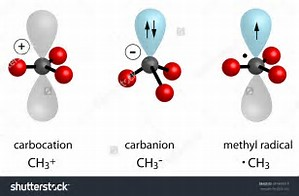
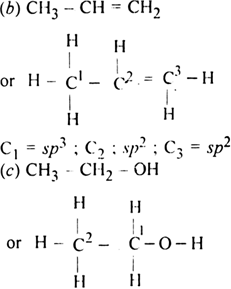
What is the hybridization of the carbon atom in each of the following CH_3^+, CH_3^-, CH_3? | Homework.Study.com

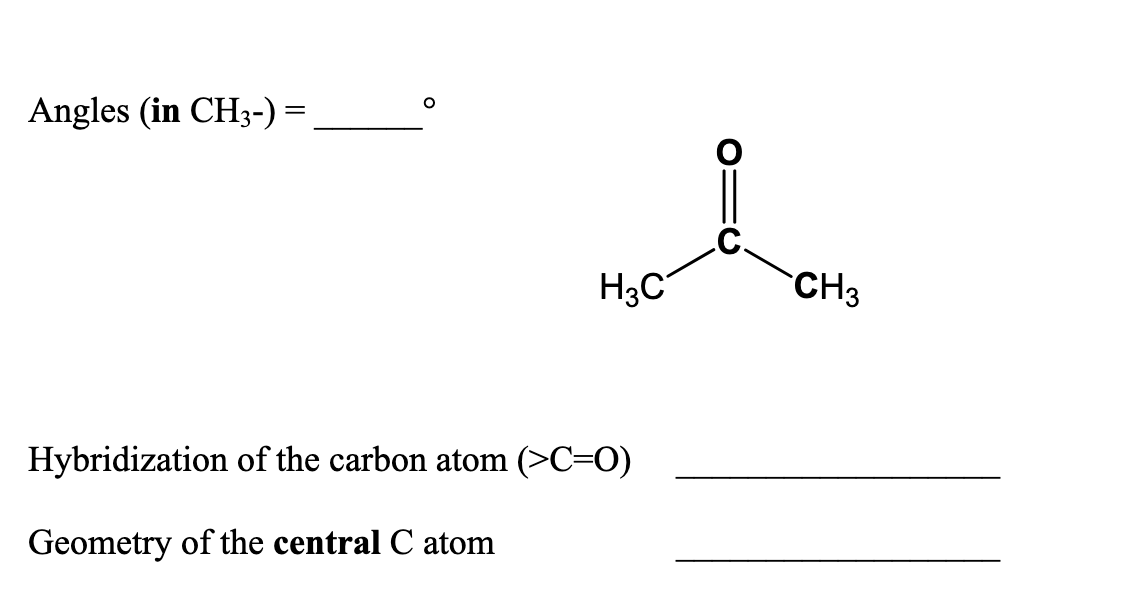
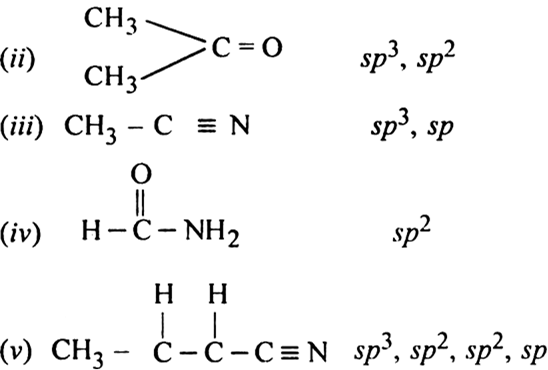
Doubt: What is the type of hybridisation of each carbon in the following compounds? (CH3)2CO , HCONH2 Chapter: Organic Chemistry - Some Basic Principles And Techniques - Subject: Chemistry - Course: NEET
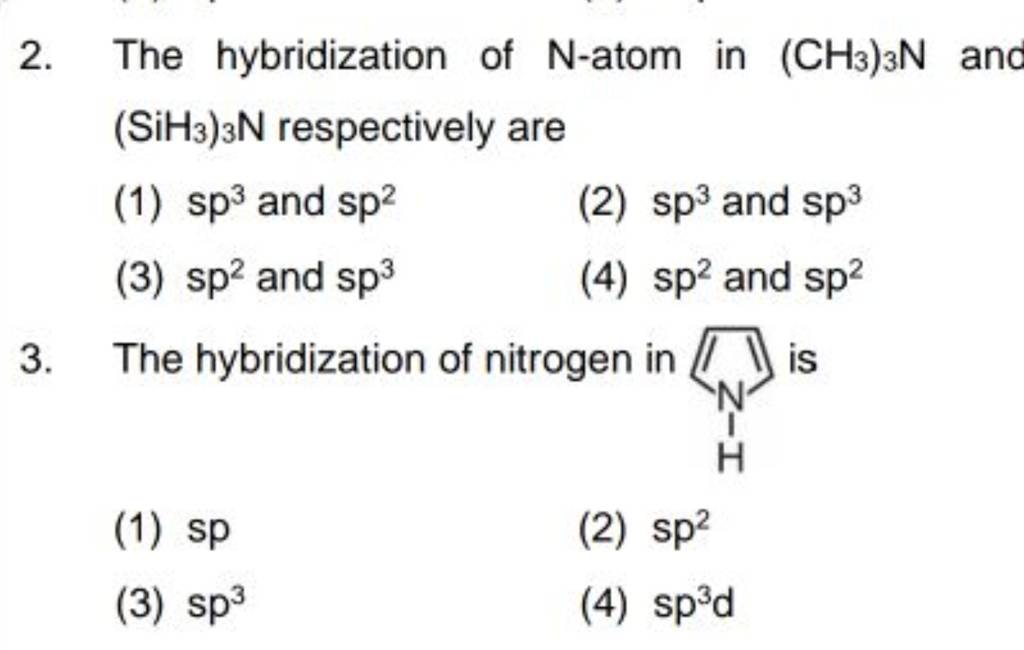
10.What is the hybridization of Oxygen molecule in the following : 1. CH3 O CH=CH2 2. CH3COCH3 [Propanone]




![Solved] The hybridization of the central carbon in CH3C≡N and Solved] The hybridization of the central carbon in CH3C≡N and](https://storage.googleapis.com/tb-img/production/20/10/F1_Utkarsha_Madhu_20.10.20_D3.png)