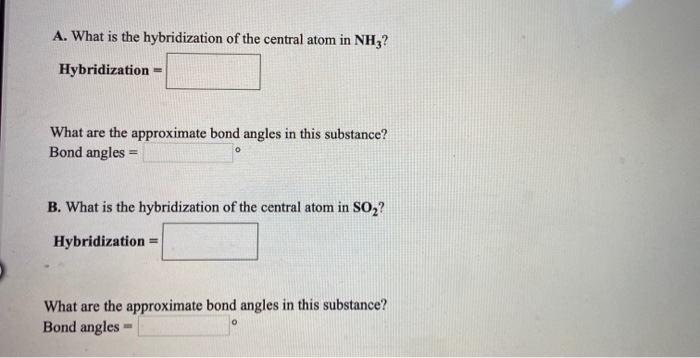
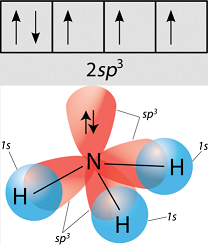
Draw the molecular shape of NH3 and indicate which orbitals overlap with each other to form the bonds. | Homework.Study.com

What type of hybridisation is associated with N in NH3? What is the expected bond angle in NH3? - Zigya

Chemistry - Molecular Structure (39 of 45) Hybridization with Free Electron Pair - Ammonia - NH3 - YouTube

Draw the molecular shape of NH3 and indicate which orbitals overlap with each other to form the bonds. | Homework.Study.com

Powerful Orbital Hybridization of Copper–Silver Bimetallic Nanosheets for Electrocatalytic Nitrogen Reduction to Ammonia | Inorganic Chemistry
Why does nitrogen (in NH3) have to be hybridized? Doesn't it already have 3 valence electrons in the 2p orbitals to pair up with the 3 electrons from the 3 hydrogen atoms? - Quora
Explain the shape of NH3 molecule using hybridization. - Sarthaks eConnect | Largest Online Education Community

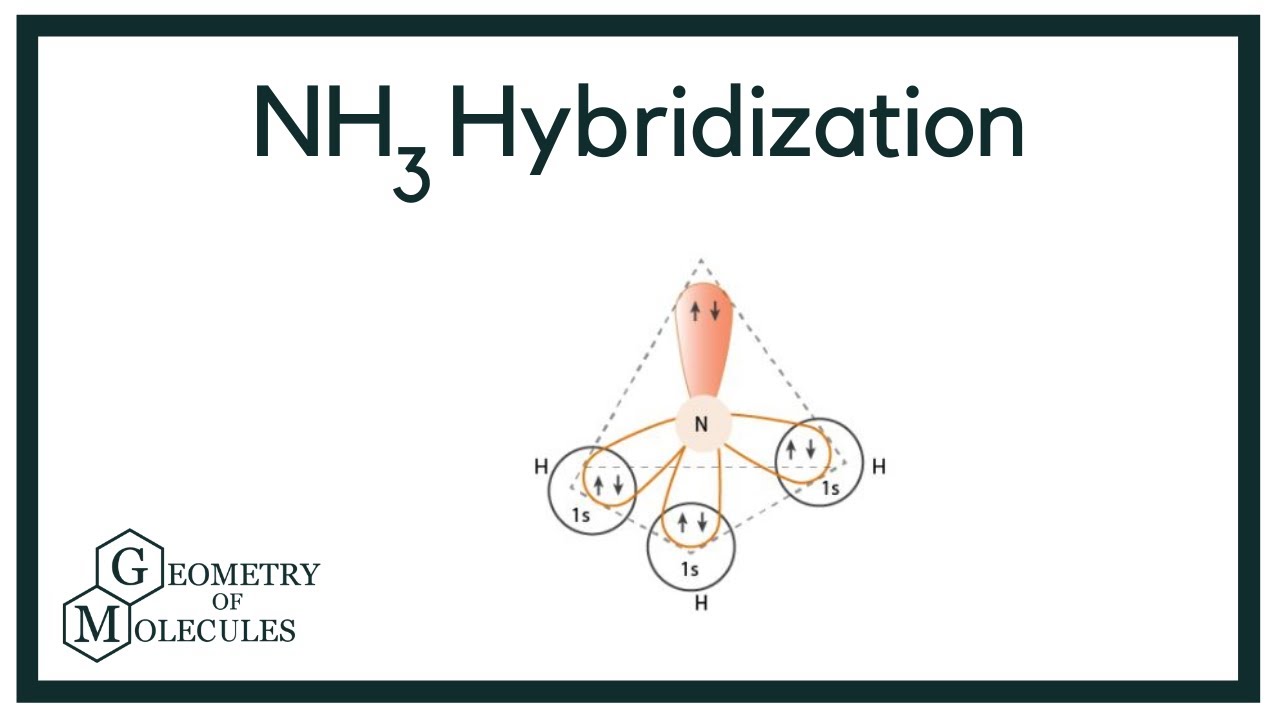
Experimental evidence suggests that the nitrogen atom in ammonia, NH3, has four identical orbitals in the shape of a pyramid or tetrahedron. Draw an energy-level diagram to show the formation of these
.webp)
Sp3 Hybridization: Definitions, Examples, Properties, Electronic Configuration, Sigma Bonds, and Differences | CollegeSearch






![why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE why cant [Ni(NH3)6]^2+ have dsp3 hybridization then? : r/CBSE](https://preview.redd.it/why-cant-ni-nh3-6-2-have-dsp3-hybridization-then-v0-slwfvbv55ria1.png?width=640&crop=smart&auto=webp&s=f4e658df50fc25da185b4ccd9590bfb69fc4c902)