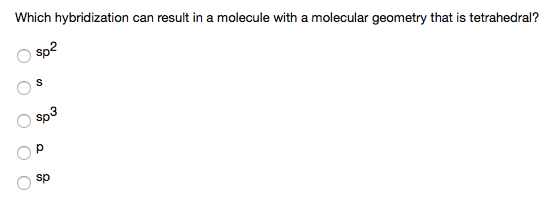inorganic chemistry - Hybridization of orbitals and forming of bonds in the nitrogen dioxide molecule - Chemistry Stack Exchange

Is there a picture of how the atomic orbitals are overlapped in nitrate and what type of sp hybridization does N and O have? - Quora
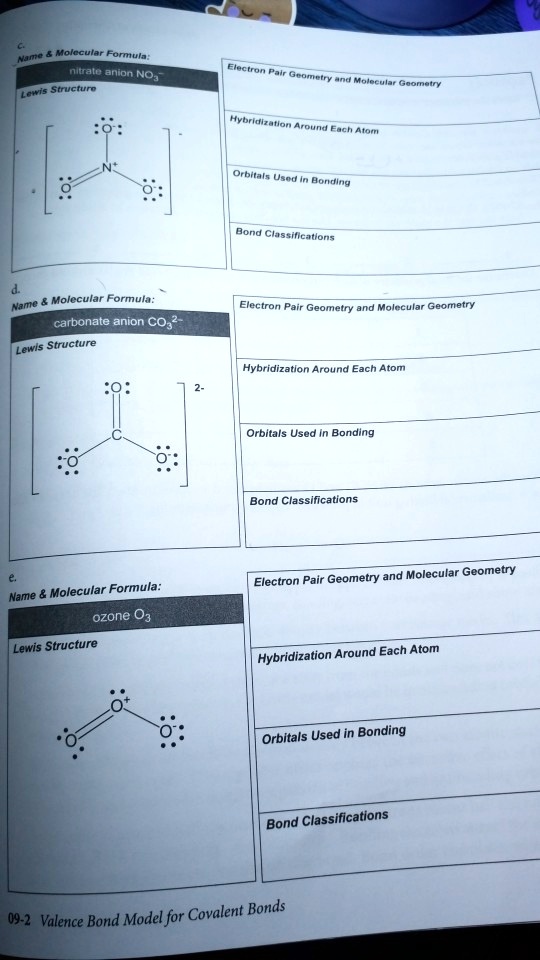
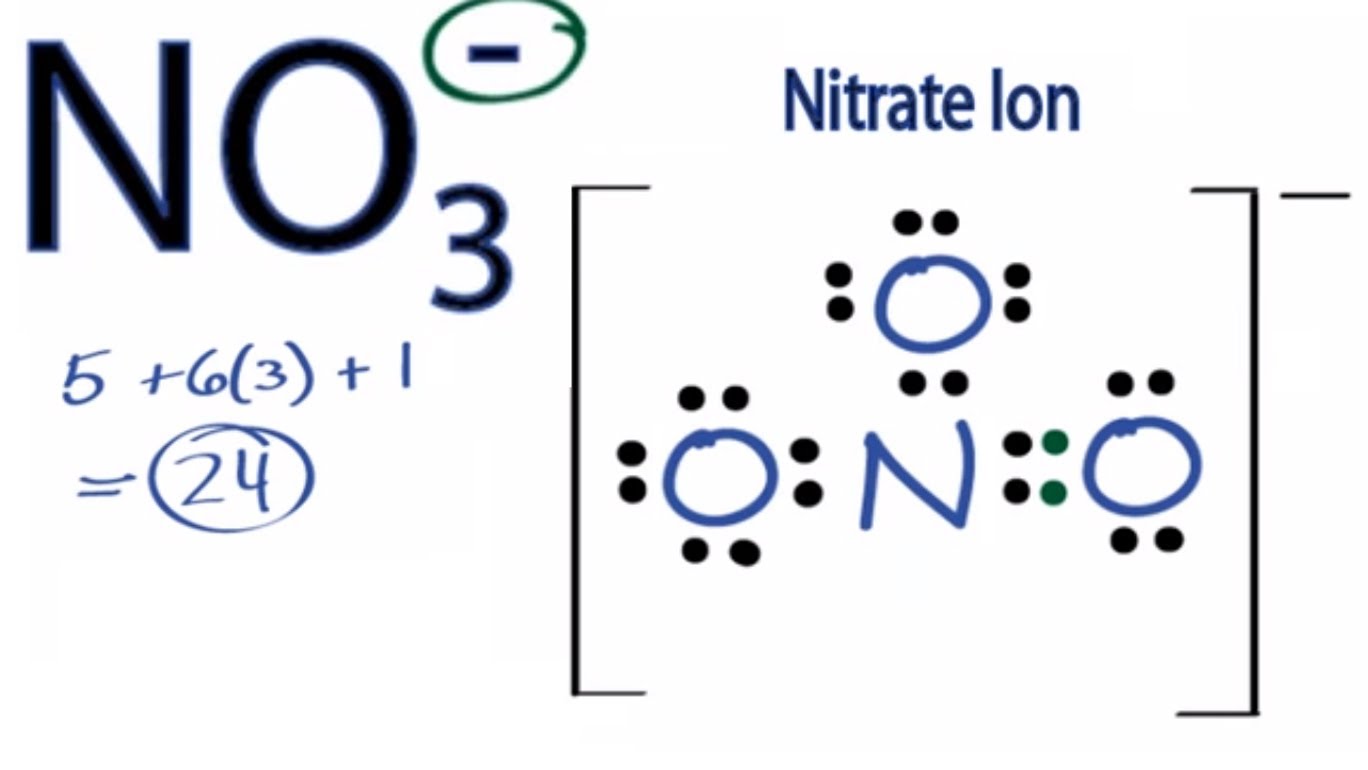
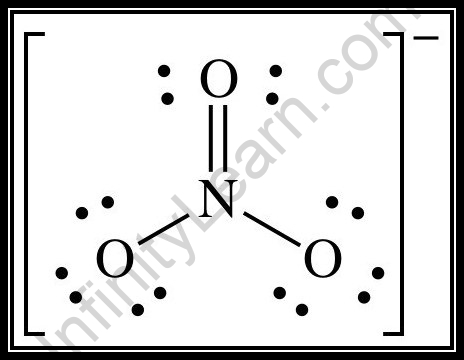
SOLVED: Molecular Formula: nitrate anion NO3 Structure Molecular Geometry Valence Electron Around Each Atom Orbital Hybridization According to Bond Classifications Molecular Formula: carbonate anion CO3 Structure Electron Pair Geometry and Molecular ...

inorganic chemistry - Hybridization of oxygen in Nitrate ion and the location/bond of nitrogen's lone pair - Chemistry Stack Exchange
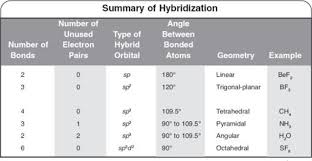
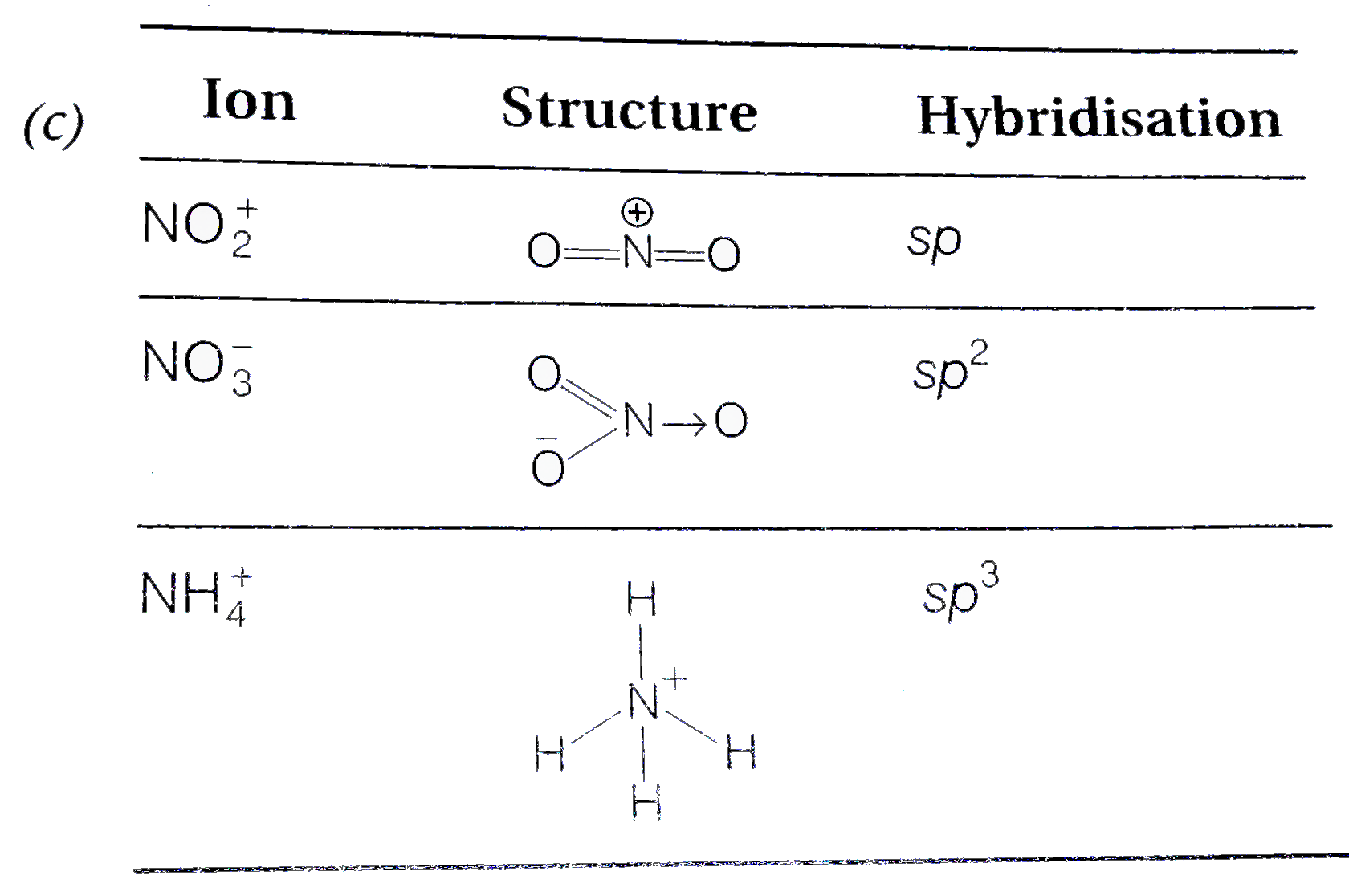
The hybridisation of orbitals of N atom in NO3^–, NO2^+ and NH4^+ are respectively - Sarthaks eConnect | Largest Online Education Community