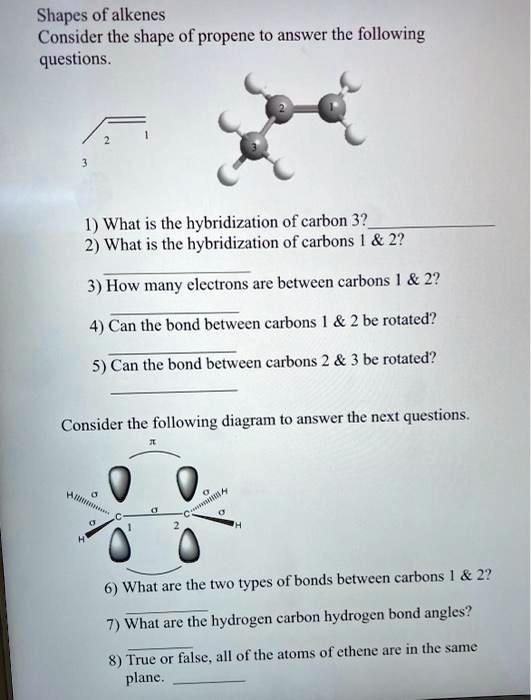
How to draw a line-bond structure for propene, CH3CH≡CH2? What is the hybridization of the orbitals on each carbon - Quora

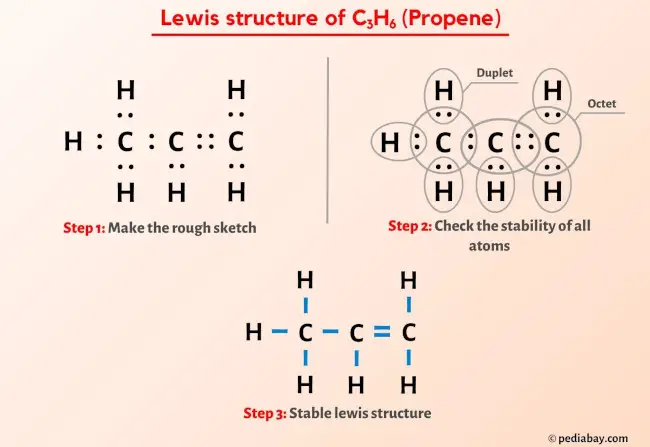
Propene C3H6: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure – infographic
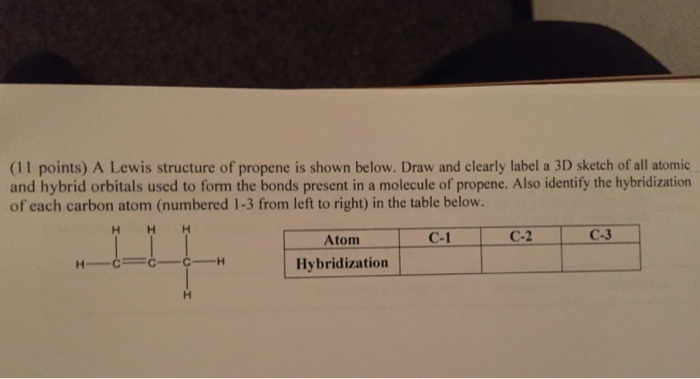
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...
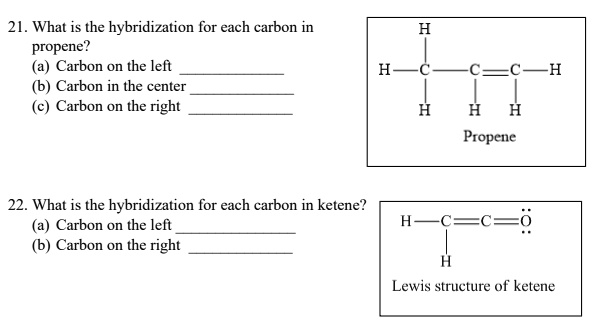
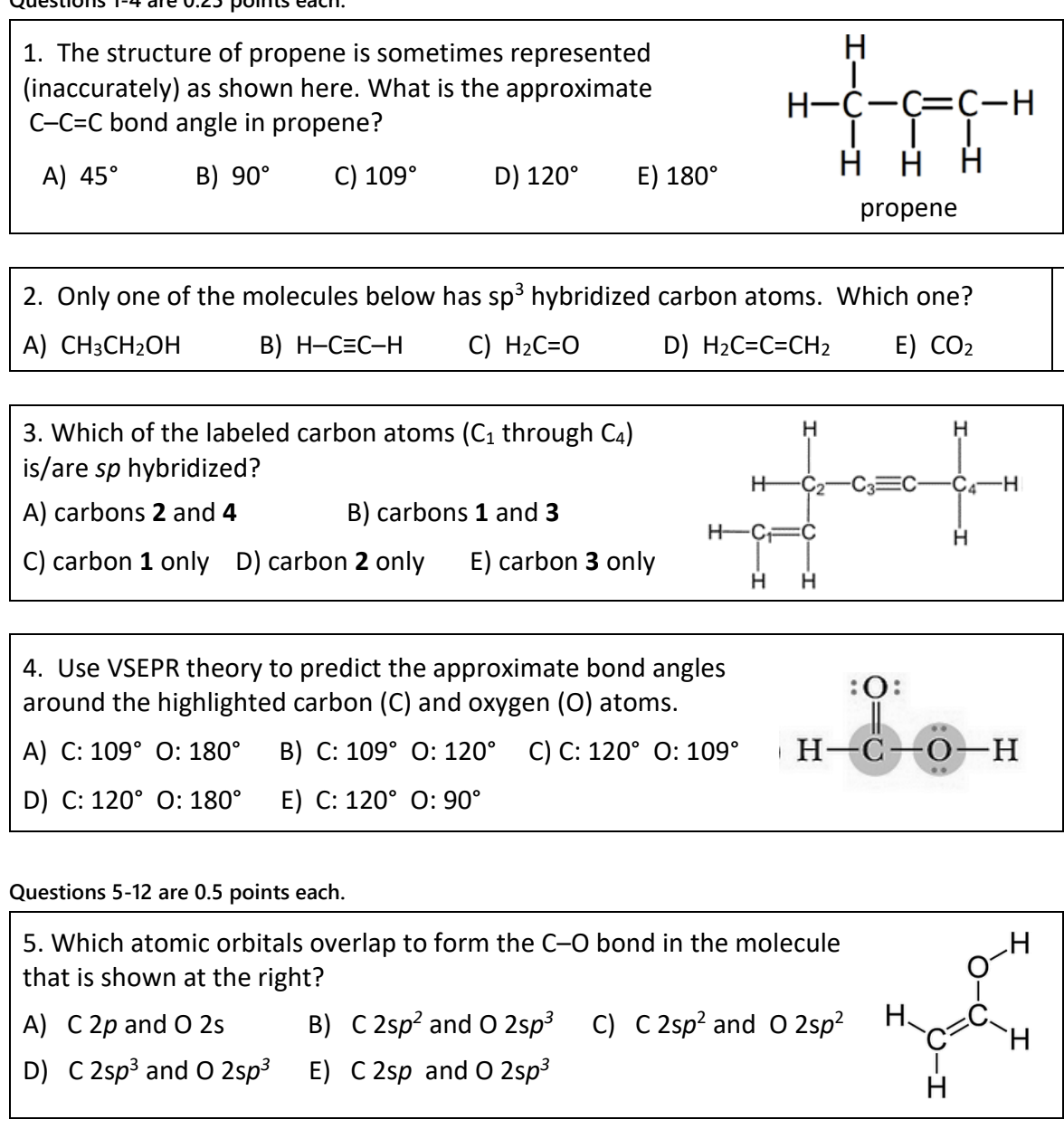
SOLVED: 21 What is the hybridization for each carbon in propene? Carbon on the lefi Carbon in the center Carbon on the right C=C–H H Propene 22 What is the hybridization for

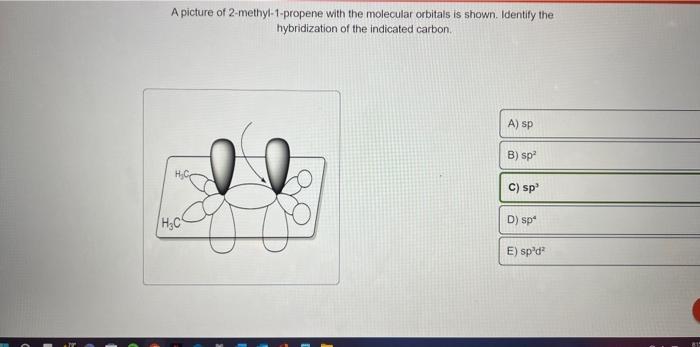
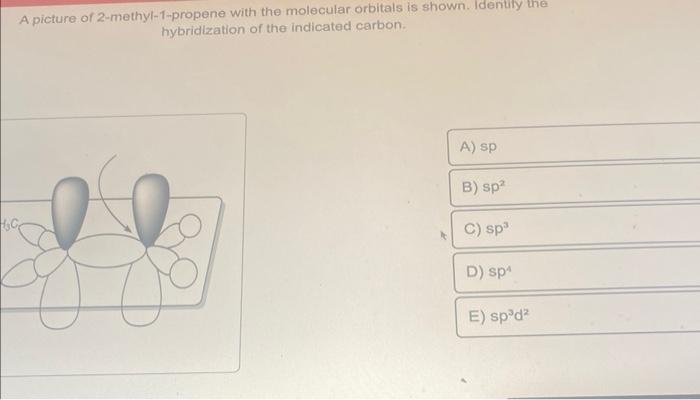
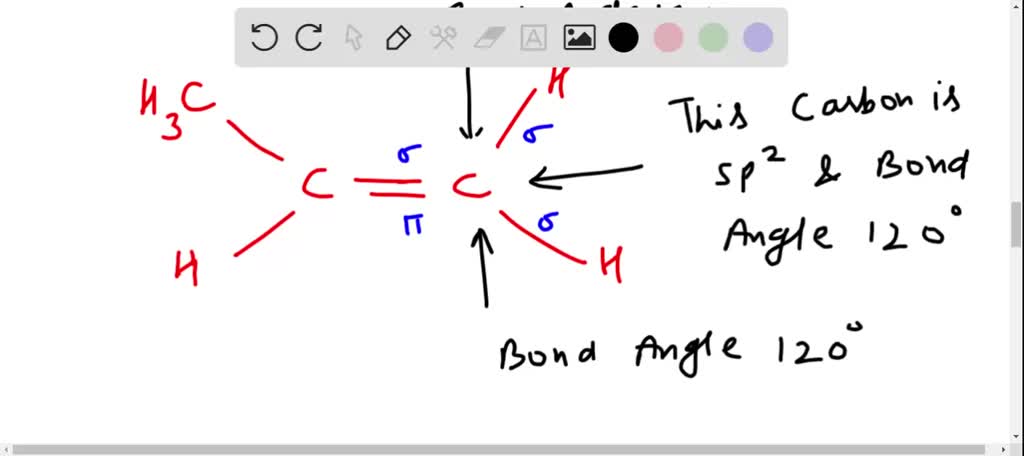
How exactly is this carbon considered sp2 hybridized when it has 3 substituents and a lone pair? : r/OrganicChemistry
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...

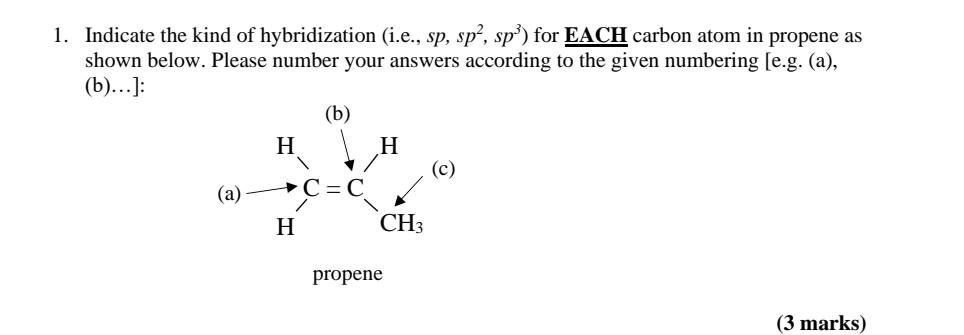
Draw the molecular shape of propene and determine the hybridization of the carbon atoms. Indicate which orbitals overlap with each other to form the bonds. | Homework.Study.com