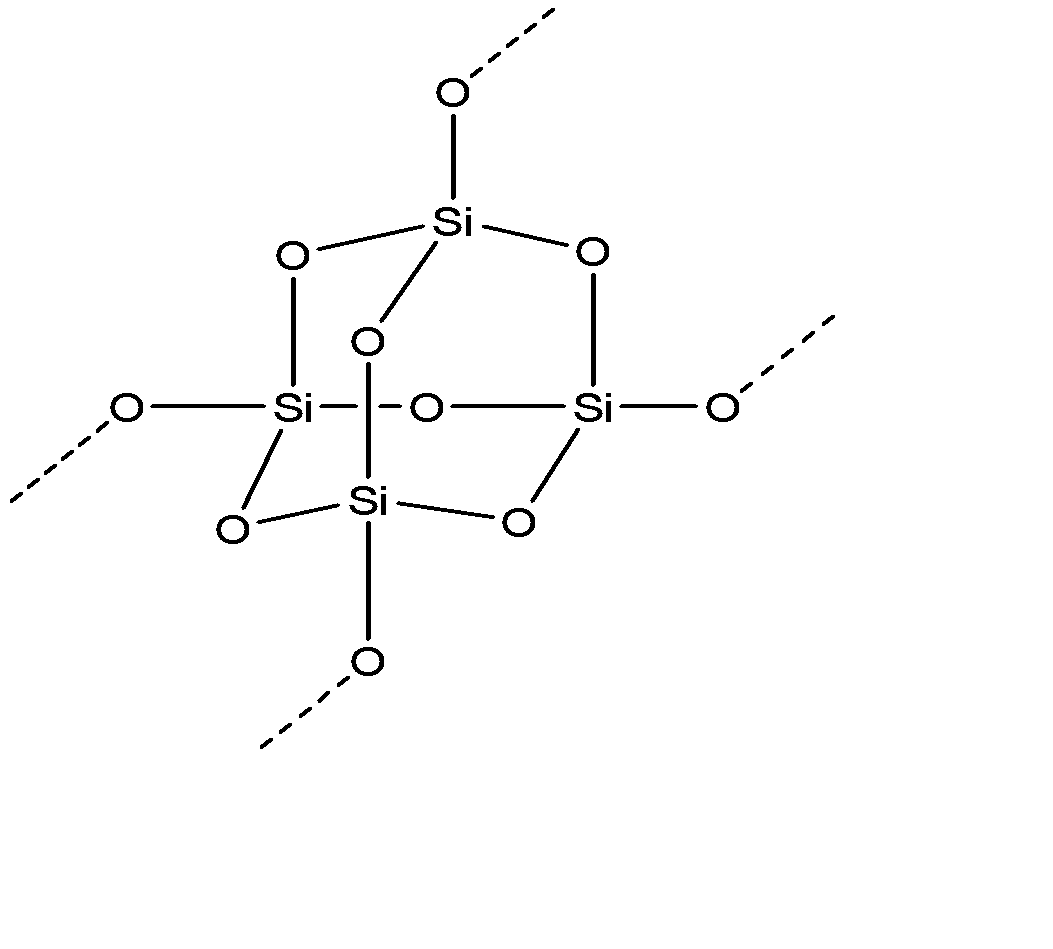
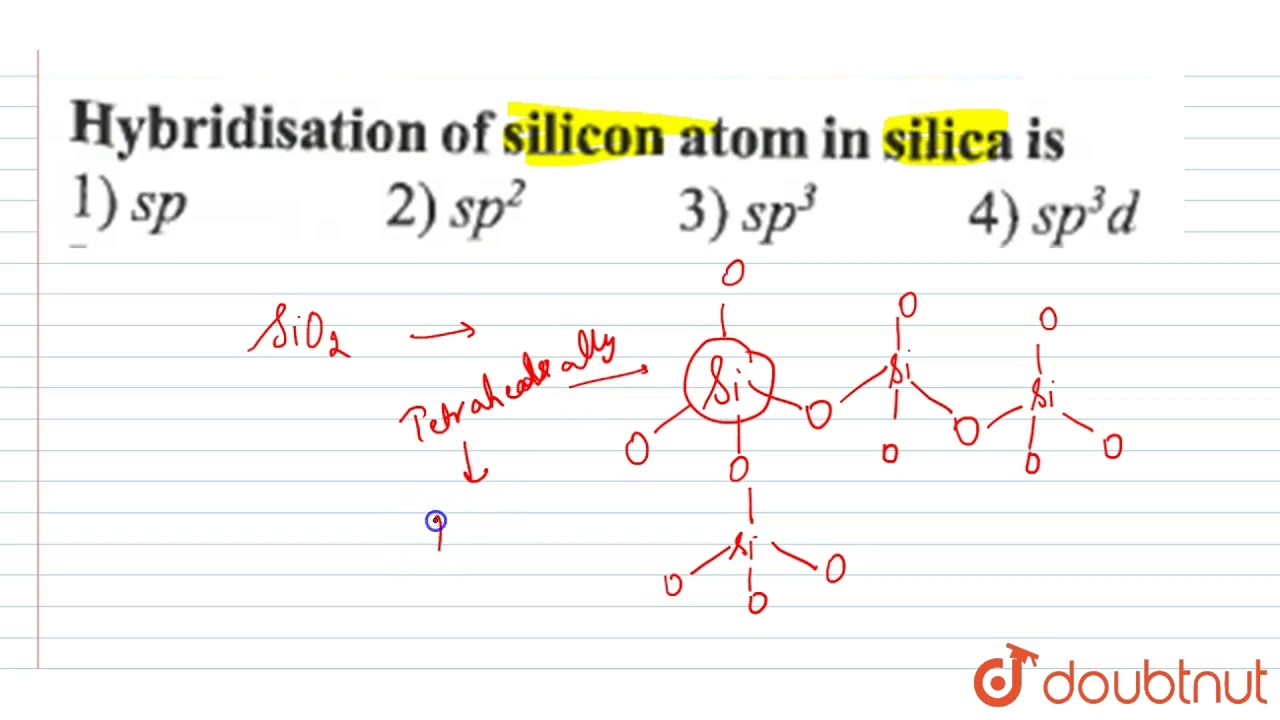
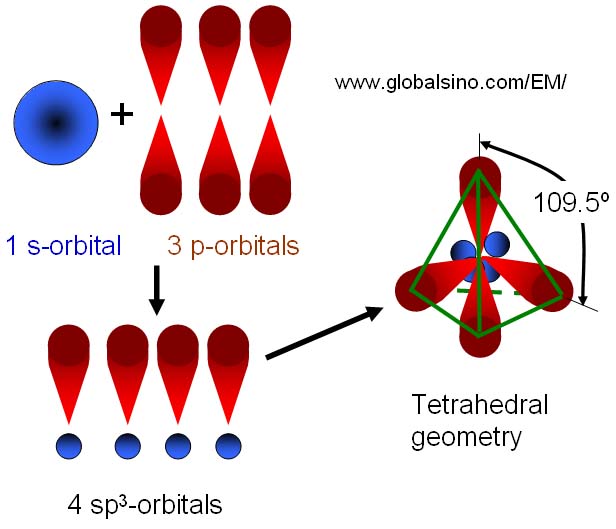
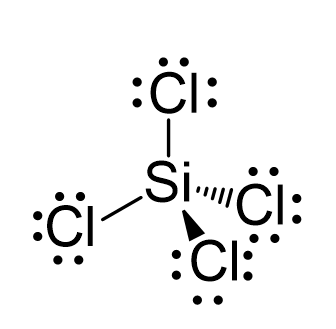
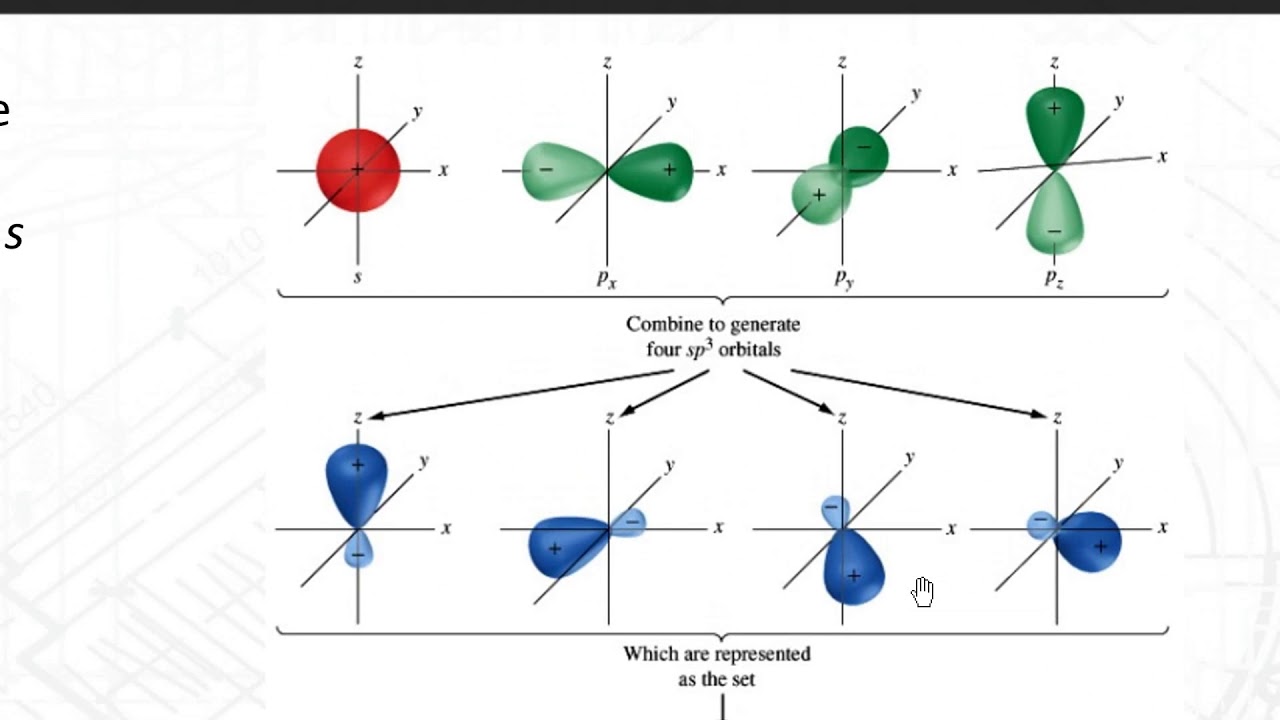
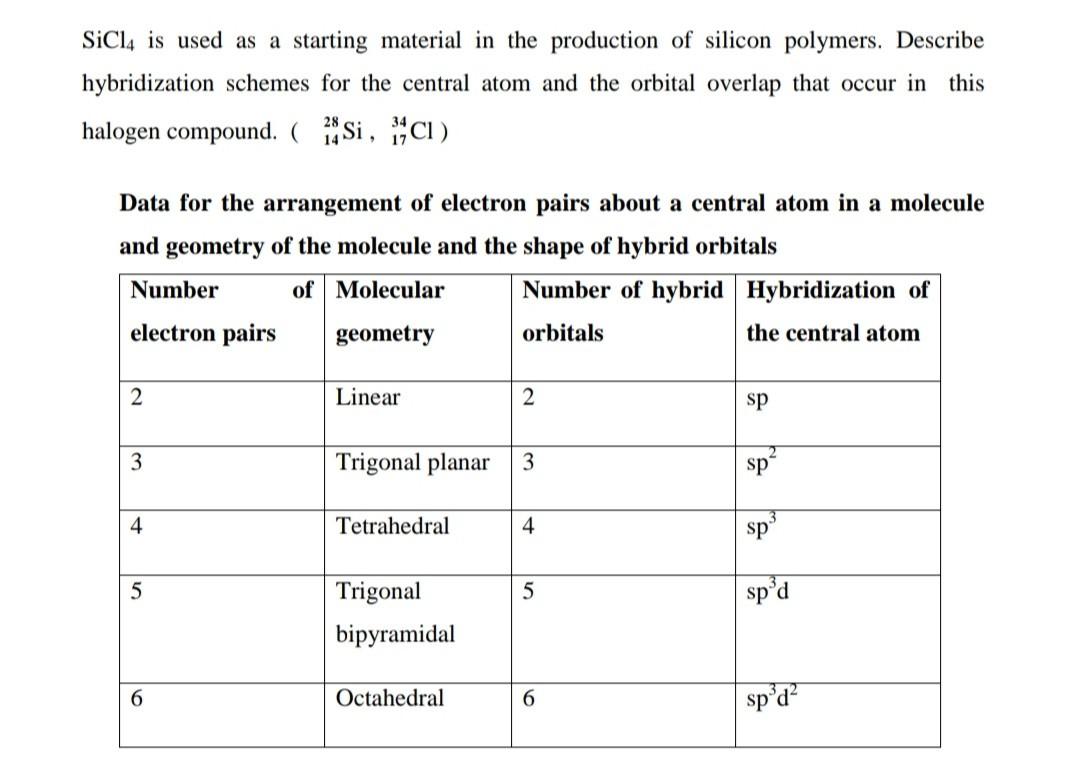
Silicon has a diamond-like structure, with each Si atom bonded to four other Si atoms. Describe the bonding in silicon in terms of hybrid orbitals. | Homework.Study.com

Silicon tetrafluoride, SiF_4, is a colorless gas formed when hydrofluoric acid attacks silica (SiO_2) or glass. Describe the bonding in the SiF_4 molecule, using valence bond theory. | Homework.Study.com

a) sp 2 hybridized planar silicene (b) buckled silicene having sp 3... | Download Scientific Diagram

physical chemistry - What are the height and width of the large and small nodes of the sp3 hybridized orbitals of carbon and silicon? - Chemistry Stack Exchange
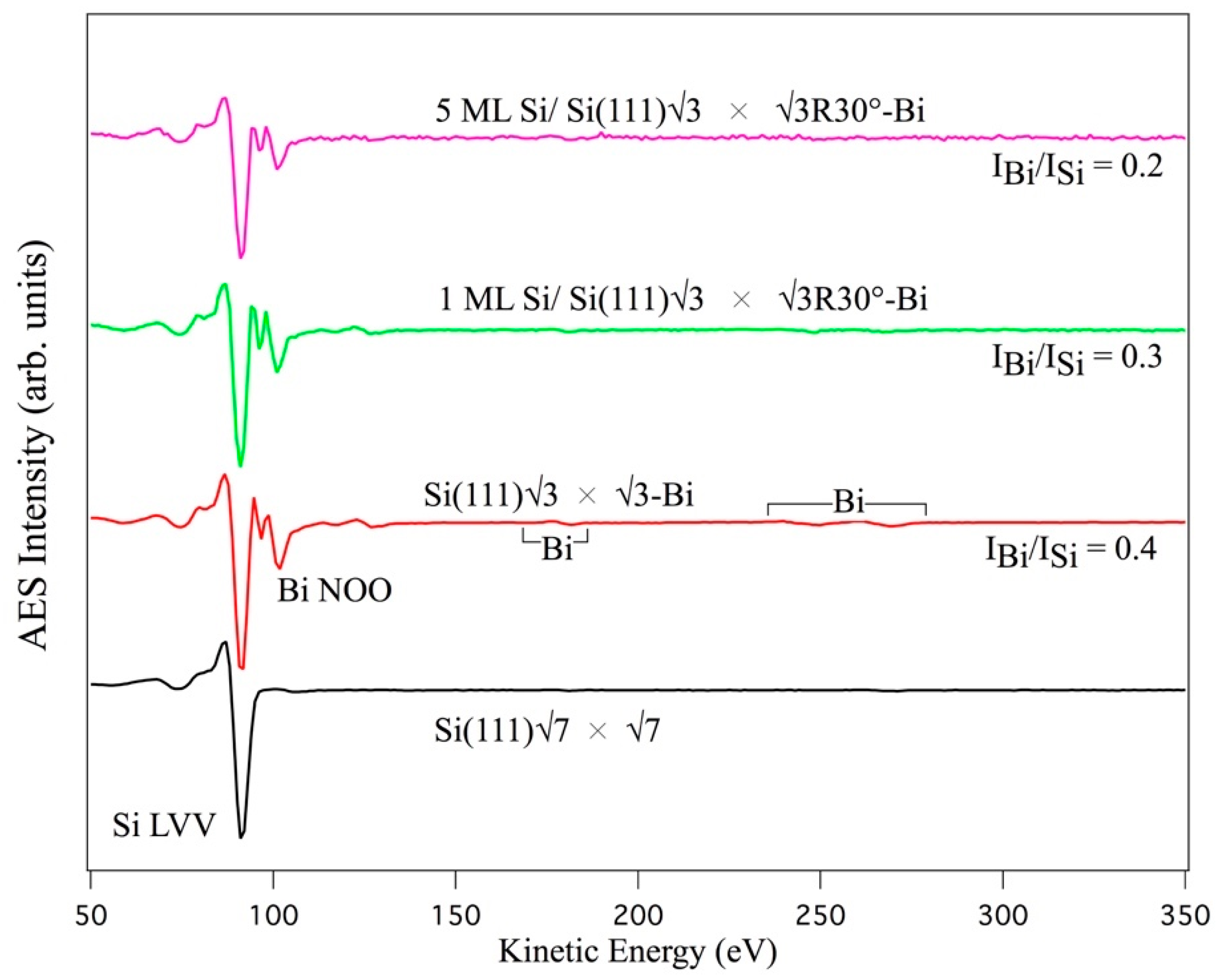
Materials | Free Full-Text | Evidence of sp2-like Hybridization of Silicon Valence Orbitals in Thin and Thick Si Grown on α-Phase Si(111)√3 × √3R30°-Bi